AMS Trace, Test & Treat Platform
Overview Trace, Test & Treat Platform
AMS is committed to flatten the curve of the COVID-19 pandemic through its ‘Trace, Test and Treat’ platform which promotes worldwide eradication of the virus.
In its trace stage AMS designed and created two COVID-19 booths:
- Infection Disease Diagnostic Sampling Booth (IDDSB)
- Mass Screening Swab Booth (MSSB)
 The IDDSB is a pressure-tested booth that can be integrated with a hydrogen peroxide bio-decontamination system for the main purpose of mass testing symptomatic person under investigation (PUI) and person under monitoring (PUM) with COVID-19 like symptoms, it can also be used for repeat testing of COVID-19 positive patients, including sputum / lavage (saliva), thus ensuring protection of the HCW and the environment. On the other-hand, MSSB is a non-pressure decay tested booth designed to diagnose asymptomatic person under investigation (PUI) and person under monitoring (PUM) in a short span of time while eliminating the risk of front-liners contracting the virus.
The IDDSB is a pressure-tested booth that can be integrated with a hydrogen peroxide bio-decontamination system for the main purpose of mass testing symptomatic person under investigation (PUI) and person under monitoring (PUM) with COVID-19 like symptoms, it can also be used for repeat testing of COVID-19 positive patients, including sputum / lavage (saliva), thus ensuring protection of the HCW and the environment. On the other-hand, MSSB is a non-pressure decay tested booth designed to diagnose asymptomatic person under investigation (PUI) and person under monitoring (PUM) in a short span of time while eliminating the risk of front-liners contracting the virus.
With the booth’s programmed pressurization (MSSB : +ve for HCW and -ve for PUI booth and for IDDSB : -ve) strict airflow regime and partnered with stringent SOPs, AMS makes easy a safe and efficient mass testing in each community to trace and diagnose all asymptomatic carriers and Person Under Investigation with COVID-19 like symptoms to prevent further spread of the virus.
AMS Turnkey Treatment Center (TTC)
The Need for AMS Turnkey Treatment Center (TTC)
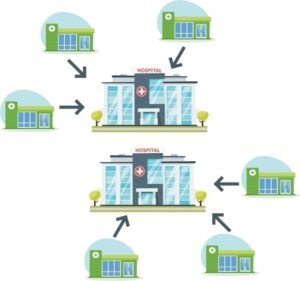 Based on the dynamics of the COVID-19 pandemic, response interventions should be done to effectively control and mitigate the disease. As countries are experiencing different severity levels of the said disease, corresponding resource requirements (isolation, oxygen therapy, mechanical ventilation) should be tailored to address the pandemic immediately. During the first three phases of case severity, a patient’s journey can be represented in Figure 1.
Based on the dynamics of the COVID-19 pandemic, response interventions should be done to effectively control and mitigate the disease. As countries are experiencing different severity levels of the said disease, corresponding resource requirements (isolation, oxygen therapy, mechanical ventilation) should be tailored to address the pandemic immediately. During the first three phases of case severity, a patient’s journey can be represented in Figure 1.
 In the phase of pandemic, the goal is to mitigate its impact & reduce its incidence, morbidity, mortality & disruption. However, the evolution of this pandemic caused an alarming exhaustion of the public health system and biomedical supplies in some countries. In order to protect this from further expanding, it is a must to centralize specific case management to simplify referrals across hospitals, primary healthcare centers and severe acute respiratory infection (S A R I) treatment centers (as shown in Figure).
In the phase of pandemic, the goal is to mitigate its impact & reduce its incidence, morbidity, mortality & disruption. However, the evolution of this pandemic caused an alarming exhaustion of the public health system and biomedical supplies in some countries. In order to protect this from further expanding, it is a must to centralize specific case management to simplify referrals across hospitals, primary healthcare centers and severe acute respiratory infection (S A R I) treatment centers (as shown in Figure).
About AMS TTC
 Many lock down cities, refugee camps, outbreak clusters or states may not have nearby hospital or diagnostics.
Many lock down cities, refugee camps, outbreak clusters or states may not have nearby hospital or diagnostics.
AMS TTC adapted from WHO Severe Acute Respiratory Infection (SARI) guidelines provides a modular easy to assemble and disassemble treatment center for immediate relocation to next cluster turnkey solution.
Our integrated approach brings out complete solution to point-of-need rather that point-of-care in a decentralized manner to supplement and provide fast response to countries.
AMS TTC total containment is provided to protect healthcare workers and to greatly reduce health system exhaustion.
Key Features
- Simple to deploy for temporary testing operations
- Exposure risk elimination for provider patient through labelled distinctive zones
- Uninterrupted testing cycles for diagnosis and treatment acceleration
- Modular partition system with advanced ventilation as recommended by WHO
- Available in tent, drive through or containerized (for rapid deployment) configuration.
